Intermediate or Western Polypody Polypodium interjectum
Mosaic Trust, Austerfield
Intermediate polypody Polypodium interjectum is one of three polypodies found in Britain. The other two are Polypodium vulgare and Polypodium australe. For the characteristics of all three UK Polypodium species, have a look at the key/review on page 153 in the
Field Studies Council AIDGAP Fern Guide. According to the South Yorkshire Plant Atlas, Polypodies are very local with only four sites in the Doncaster MB.
Polypodies are species of ferns which are pteridophytes, non flowering vascular plants with true roots, stems and leaves. The major difference between ferns and flowering vascular plants is that they reproduce by spores rather than seeds. Their reproductive structure evolved long before the flowering vascular plants.
The showy fronds are the parts of the life cycle of ferns which are observable in the field and, when mature, bear spore- production organs called sporangium. In photograph Fig 1, below, you can see the plant is in the spore bearing stage from the circular raised areas on the front of the fronds and more clearly from the orange- brown clusters (sori) showing on the underside.
Below, in photograph Fig 2, you can see a section of a fern frond showing a closer look at the branches of the frond, called pinna. Along each side of the midrib there are clusters of circular capsules which are the spore bearing structures called sporangium. Together they are called sporangia and a cluster of sporangia are referred to as sori.
A closer look, as in photograph Fig 3 below, will reveal that they are in different stages of maturity, some having released their spores, which can be seen as tiny yellow specks on the surface of the frond.
To explain spore dispersal further you need to look more closely at the cell structure of the spore capsule ,referred to as the sporangium. On the outer rim of the capsule is the annulus, a belt of dead, water-filled cells with thickened walls which stretch about two thirds round the capsule. (in the photograph below looks rather like a ladder). As the sporangium dries, evaporating water is drawn from the cells of the annulus causing the cells to shrink. The thick inner walls don’t collapse but the outer walls start to buckle causing the curved surface of the annulus to contract. The weaker cells opposite are ripped apart and the wall of the sporangium ruptures. Meanwhile the two ends of the annulus slowly straighten so that the two ends of the recurved annulus almost meet. Nearly all the spores are carried on the free end in a cup formed by the lateral walls of the sporangium. Suddenly the annulus snaps back into its original position and in the process catapults the spores from the capsule. This ejection mechanism allows the tiny spores to be dispersed over a larger area.
The number of cells in the annulus varies in the different species and is used as an identification feature though a selection of sporangium of the same species would have varying numbers of cells. The two photographs below, photograph Fig 3 and Fig 4 , were taken using a Samsung Galaxy S10 phone in an adapter attached to a microscope. If you look closely you can see the annulus both from the back, front and side of the sporangium. However, even at this level of magnification you can see how hard it is to count the cells of the annulus and simply counting the cells of one annulus would not be sufficient to identify the species. I think looking at other features might be more useful. For more information on the structure of the sporangium and the number of cells recorded in the annulus of the three polypodies refer to Britain’s Ferns by James Merryweather.
References : Wikipedia.
Spore discharge of Common Ferns A.L.King 1944.
Britain’s Ferns by J.Merryweather.
Pemphigus spyrothecae on Populous nigra and hybrids
Part 1: Position, formation and structure of spiral galls
Pemphigus spyrothecaea are woolly aphids in the hymenopterous division of the order Hemiptera, in the subfamily Aphidoidea, which consists of insects with sucking mouthparts.
Position of galls.
The galls induced by Pemphigus sprothecaea are spiral galls and appear on the petiole of Black and Lombardy Poplar leaves. They often occur on multiple leaves close together and are instantly obvious when examining a tree as you can see here in Figure 1.
Stem mothers or fundatrices, developed from overwintering eggs, search for the best galling sites on the petioles of Poplar leaves, often competing with others and using their back legs to kick others out of the way. Once the site is located, the fundatrix pierces the petiole with its stylets and as it penetrates tissues on the way to the phloem it injects saliva into the plant and also into the phloem element. The aphid carefully chooses where to inject the saliva to produce a complex structure that will completely enclose the aphid and its descendants. The aphid even controls the subsequent opening so that its descendants can escape and move elsewhere.
Margaret Redfern, in her book “Plant Galls”, states that “Not all leaves are equally attractive to the fundatrices. The best leaves being those that grow rapidly , are destined to become the largest, that attract the greatest share of nutrients (ie become the strongest nutrient sink ) and that contain the lowest concentration of chemical defences. Small leaves with galls often drop early before the aphids have left and that can mean the death of the whole clone.” After penetration, the fundatrix then probes backwards and forwards causing the beginning of torsion. The pattern of probing helps to determine the shape of the gall and the site of the future aperture. Swelling and twisting of the petiole by the feeding nymphs leads to the formation of hollow galls that protect the nymphs from the weather and predators.
The growth of the gall in the petiole occurs by cell multiplication in petiole meristematic tissues and is a response of the host to the nymph feeding ( National Library of Medicine) The exact substance which causes a gall to form is still a mystery. Aphid saliva contains many compounds some of which could be cecidogenic (gall causing ) The two most likely substances are the plant growth hormone IAA, either transmitted in the saliva or forming in the plant by oxidative transamination of tryptophan by enzymes in the saliva or free amino acids in the aphids saliva. ( Karen . L. Alton )
The gall causers induce the petioles to swell, flatten and spiral to form smooth galls which are initially green but when mature they can be tinged with red. They can develop as single, double or even triple galls joined together or separate. They can be close to the blade or further down the petiole. Morphologically the galls consists of epidermal tissue on the outside and a hollow space harbouring the aphids.
On observing the position of the galls in a sample of 20 leaves , 5 were induced where the petiole is attached to the blade (see examples in Figure 2.), 8 were between 0 and 10mm from the blade and 7 were further than 10mm away from the blade, some almost at the twig end of the petiole, as in Figure 3 below..
However my sample was quite small and Karin L. Alton, in a study carried out at Nottingham University, found that most stem mothers located their galls at the top of the petiole, closest to the blade of the leaf. She also determined that gall position didn’t affect the number of offspring but that larger leaves or shoots supported galls containing a higher number of offspring and a lower rate of gall failure. Compson et al. 2011 found that petiole twisting and abnormal outgrowths ( galls ) lead to the interference of transport of water and primary and secondary metabolites and mechanical restriction on vascular tissue. Rosen and Percy 1993 states that the presence of galls can compromise the wind -driven rotational lamina movements ie fluttering and can lead to premature leaf drop during strong winds due to excessive drag.
Sometimes two galls develop some distance apart on the same petiole as in Figure 4 below.
Sometimes torsion is induced in a petiole but for some reason the gall doesn’t fully develop, as you can see in Figures 5, 6 and 7 below.
Other times two galls develop very close together and appear to be fused as in Figure 8, below left.
In all the galls, the emergence slit through which the aphids leave the gall is obvious. See Figure 9, below right.
This slit is initially open but by late April is closed and doesn’t open again until late August when winged sexuparae (parents of sexual aphids) emerge through the furrow between the coils of the gall. Figure 10, below shows a fallen leaf bearing a gall with a hole in its side, created by a predator. It’s not clear whether the attack would have taken place while the leaf was still on the tree but given the fact that most aphids leave before leaf fall it’s highly likely that it took place at that time.
References
The Biology of Pemphigus spyrothecae galls on Poplar leaves: PhD thesis by Karen L Alton Jan 1999
Plant Galls by Margaret Redfern
National Library of Medicine Trees
Aphids on Deciduous Trees T. Dixon and T. Thieme.
Pemphigus spyrothecae on Populus nigra and its hybrids
Part2: Life cycle of gall causers
The insect which causes the spiral gall on the petiole of Poplar leaves is a woolly aphid called Pemphigus spyrothecae and its life cycle follows the basic pattern of aphid development. Aphid lifecycles can be described as holocyclic in which a cyclic path occurs with aphids reproducing sexually in the autumn to produce an overwintering egg and pathenogenesis during spring and summer. Deciduous tree dwelling aphids like P. Spyrothecae exist in three morphs, wingless egg laying females, pathenogenetic females ( which give birth to live females ) and winged males. They are non -host alternating species which means the two generations occupy the same host, either in the gall or free living on or in the bark.
Lifecycle in more detail
Egg-Egg
Very few tree-dwelling aphids lay their eggs close to the terminal buds. Most are laid in pores in the bark of twigs or in crevices in the thicker or older bark on the main trunk at considerable distances from the buds, especially those in the upper canopies of mature trees. Possible advantage is that it affords eggs protection from birds.
Egg hatch occurs at or shortly after bud burst. Such close synchronization results in highly fecund adults, those hatching earlier or later are less fit. Few of those that hatch before bud burst survive because there is no expanding bud on which they can hide from birds or shelter from heavy rain.
The eggs hatch in the Spring and give rise to nymphs which develop into adults of the first generation known as fundatrices. These female wingless aphids then search for a suitable site to induce a gall by piecing the phloem with their stylets and injecting saliva into the plant. When they are in a favourable position to do so they defend the site from all newcomers by kicking and shoving with their hind leg. Even after starting a gall, the fundatrix can still be displaced while the gall is open. A proportion of females die while attempting to initiate a gall.
After penetration, the fundatrix then probes backwards and forwards causing the beginning of torsion. It plugs into a phloem sieve tube, moults several times, grows into an adult and lays large numbers of unfertilised eggs which develop into females, in the process called parthenogenisis.
Most of the phloem sap ingested by aphids is excreted in liquid form as honeydew which left as a liquid could contaminate or even drown the aphids in a closed gall. So all gall causing aphids produce wax to parcel and transport the honeydew. Special cells concentrated in the upper epidermis of the abdomen and around the anus produce wax so that as each drop of honeydew emerges from the anus it is coated in wax strands and further coated with wax powder as the drop rolls around in the gall. These form “marbles” that prevent the honeydew from wetting the aphids and the lining of the gall. Being spherical they can easily be pushed out of the gall.
Figure 1 and Figure 2 above show part of the contents of a spiral gall opened on the 12th of September showing the “marbles” of honeydew. The contents fell out and scattered as seen in the photos. (see reference to Gall 4 under Further Observations below)
In Figures 3 and 4 below, showing the insides of two other galls opened at the same time, you can see an accumulation of “marbles” at the top right of the photo, amongst the wingless and winged aphids.
Some of the first generation aphids develop into soldier aphids whose initial job is to carry out housekeeping jobs, including pushing out the wax-coated honeydew droplets and any cast skins or dead aphids, their weapons being their stylets and hind legs. They can easily roll the marbles around and out of the aperture. They lose this behaviour when they moult into the second instar. They do not feed or reproduce and are short lived, their purpose being solely to defend the colony against insect enemies and carry out the cleaning duties. They congregate round the entrance to the gall and readily attack any enemies at the entrance or on the gall’s surface and sometimes inside it. They defend the gall contents from hoverfly larvae and caterpillars stinging them with their stylets and piercing them with their armed hind legs. Soldiers can make up to 13-50% of a colony. The more vulnerable morphs that reproduce tend to be found in the safer depths of the gall. Several generations occur in quick succession until sexuparae (parents of sexual aphids) develop. They can be found from about mid -August and are at their most numerous by the end of August. When mature they leave the gall through the furrow between the coils, their departure occurring over a period of 2 to 3 months from August to October. By this time the galls have been on the petiole for about 5 months. They produce a small number of males and females on the bark which subsequently mate. The fertilised females then each lay one egg covered with wax fibres in bark crevices and the cycle begins again.
Further observations of 15 galls collected and opened on 12th September 2022 .
Out of 15 galls, four were filled with live aphids. Below are some photographs of the inside of those galls. Figure 5 below shows the contents of first gall which were lifted gently out of the hollow and show evidence of “marbles”, at least one winged individual and many wingless aphids packed together and covered with wax powder.
In the second gall containing live aphids, Figure 6 below, you can see many wingless aphids, at least one winged aphid and a very obvious accumulation of “marbles” of honeydew in the top right hand corner.
The third gall, Figure 7 above, shows a gall containing more winged individuals, many wingless ones and again an accumulation of “marbles “ in one corner. I observed the activities of the aphids for several minutes. They were very active, most of the wingless ones just moving around in the galls but the winged ones moved outside straight away and crawled around the outside of the gall. As far as I know no winged aphids flew away from the gall. On two occasions I saw what appeared to be a wingless aphid kicking a “marble” out of the gall. When I opened the fourth gall the contents fell out enabling me to take closer photos of the “marbles” referred to earlier in the text. Also to take the following photograph of a group of three winged aphids, one of which was quite clearly showing the lime green colour of its abdomen. See Figure 8 below.
The next photograph Figure 9 shows a group containing “ marbles”, shed skins of aphids, winged and wingless aphids several of which appear to have blobs of wax powder on their backs.
In figure 10 you can see two winged aphids both of which appear to have a wingless aphid on their back.
On observing two of these galls I noticed a tiny beetle scurrying away from the gall. I’m not sure whether or not it came from within the gall or was just present on the outside. See Figure 11 below.
Nine of the galls like the one in Figure 12 below, were filled with a grey mass of debris with virtually no sign of life. In the close-up of the debris in Figure 13 however you can just see a few wingless aphids.
Two of the galls were completely empty.
References
Plant Galls by Margaret Redfern.
Aphids on deciduous trees by T.Dixon and T. Thieme.
Addendum
At the YNU Entomological meeting at Potteric Carr, I discovered that the “beetle” mentioned in part two of my article about the Spiral Gall (see Fig 11) is in fact a flowerbug in the family Anthocoridae. Jim Flanagan, Sorby NHS Hemiptera recorder, thought it likely to be a specimen of Anthocoris minki minki. Jim was very interested in the find as had brought along to the meeting a presentation about the same species that he had found on a black poplar also with spiral galls at Bolton Brickyard Ponds in the Barnsley area in August. The bug is a specialist feeder on the Pemphigus aphids that induce and inhabit spiral galls on poplars. A. minki minki is probably under-recorded even though the galls are very widely reported on poplars across much of the UK. He thought that given its specialist predatory nature the specimen I saw and photographed probably crawled out from the gall as I prized it fully open.
In Margaret Redfern’s book Plant Galls she notes that ” The bug can inflict major damage, sometimes destroying the whole colony. One or two A. minki enter soon after the gall opens and can kill the entire contents of the gall before starting to feed. The dead aphids are in a relaxed state but without feeding scars or other injury and have been killed rapidly and highly efficiently.
How are they killed? Perhaps a secretion from the bug’s stink glands gasses them all at once, the secretion being particularly effective in the confined spaces of the closed gall. Anthocorid eggs are inserted into plant tissue, probably outside the gall. The first instar crawls inside, kills the aphids and completes its development inside the gall.
Analysis of a Long- eared owl pellet found at Lindholme on the 5th Feb 2022
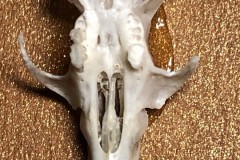
During our search for mosses on Lindholme Colin Howes discovered a collection of Long-eared Owl pellets. I collected a few and initially dissected four. In order to clean the bones of fur and hair I soaked the pellets in warm water and bleach. The Photograph 1 shows the bones found in the first pellet, hereafter referred to as Pellet A.
As you can see from the two skulls, Pellet A contained the remains of at least 2 individuals. This pellet contained examples of a variety of bones, most of which were only replicated in the other pellets so I decided just to concentrate on this one On closer examination of the skulls I discovered that both had gaps between the incisors and cheek teeth indicating that they were both rodents. Taking a closer look at the highlighted lower jaw (Photograph 2.) I could clearly see 6 root holes where the teeth had fallen out indicating that is either the lower jaw of a Wood Mouse Apodemus sylvaticus or a Bank Vole Microtus agrestis. However the shape of the upper edge of the jaw posterior to the tooth holes indicates Wood Mouse. This would be more likely anyway since one of the skulls ( highlighted in black on the first photograph ) is that of a Wood Mouse.
The skull highlighted in black (Photograph 3), shows the specimen has three teeth with knobbly cusps or crowns with worn down grinding surfaces indicating that this is an elderly specimen. In murids (mice and rats) although the gnawing front teeth (incisors) grow through life, their molars (back teeth) wear down through eating abrasive food (seeds etc.).The pattern of the root holes shown in Photograph 4, ie 4 in the second molar and 3 in the third molar indicates that it is the skull of a Wood Mouse.
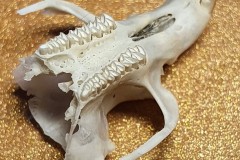
The other skull in this pellet and the skulls in the other three pellets belonged to a Field Vole Microtus agrestis. Here, in Photograph 5, you can clearly see the bold zig zag pattern on the grinding surface of the Field Voles teeth. In Photograph 6, you can see the jagged sockets left when the cheek teeth fall out and the notched incisor.
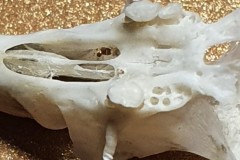
Photograph 6 of a Field Vole skull is interesting because the skull is almost intact, whereas many skulls (particularly mice ) break up either in the formation of the pellet or when they’re being dissected. On the left hand side you can see a grey bulbous structure which often falls away from the skull and is found separately . This is the auditory bullae, the part of the skull which covers the inner ear. At the very lower end of the skull you can see a large opening known as the foramen magnum. The foramen magnum allows key nerves and vascular structures passage between the brain and spine. Namely, it is what the spinal cord passes through to enter the skull. The brainstem also passes through this opening.
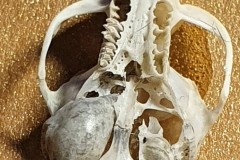
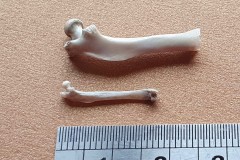
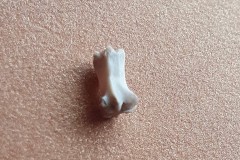
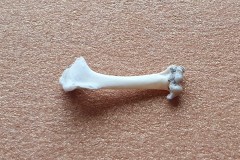
Identifying the function of other bones in the pellet was easy so within Pellet A you can clearly see examples of scapulas, arm bones, leg bones, hip bones (pelvic bones), ribs and vertebrae. Attributing them to particular species is impossible. However I did find two sets of pelvic girdles which are obviously from two different species and probably one male and the other female.
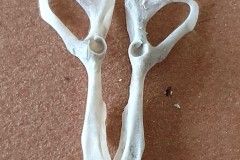
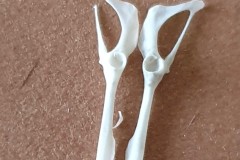
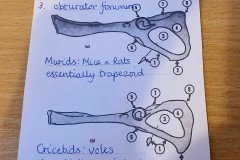
The first set, in Photograph 7, have a thicker pubis (ventral part of the hip girdle) indicating that the specimen is male and the second set in Photograph 8 have a very narrow pubis, indicating that the specimen is female. The shape of the obturator foramen (the gap in the hip girdle) is different in the two sets and this characteristic can be used to distinguish between Murids (mice and rats) and Cricetids (voles etc) See Photograph 9 from “Mammals of Britain- Their Tracks, Trails and Signs“.
The two species in Pellet A, identified from the skulls, are Wood Mouse and Field Vole so it’s highly likely that the first pair of pelvic bones, having the more trapezoid shape belong to the mouse and the second set, having an essentially semi-circular opening, belong to the vole. A few days later I decided to opened some more pellets hoping to identify the remains of species other than Wood Mouse and Field Vole. The first 4 only revealed more Field Vole skulls but the fifth pellet, hereafter referred to as Pellet B, was much more interesting because in it I found a bird’s skull, see Photographs10 and 11.
I also found a collection of limb bones and vertebra which were substantially bigger than the ones found in Pellet A, as photographs 12, 13 and 14 illustrate.
Another interesting bone found in Pellet B which is perhaps part of a tail ie a caudal vertebra is shown in Photographs 15 and 16.
And finally Photograph 17 of another bone from Pellet B, which I can’t identify.
There were no skulls or lower jaws in Pellet B making it difficult to identify the species the bones represent.
Note: According to “Mammals of Britain- Their Tracks, Trails and Signs” Field Voles are the most common prey of all owls except the Tawny Owl.
References: Mammals in Britain: Their Tracks, Trails and Signs – M.J. Lawrence, Roy Walter Brown